Animal Insulin Withdrawal: Lessons for Patient Advocates Today
19 Aug 2019, 10:51 a.m. in Global Stories by Colleen Fuller
At the end of July, the news was filled with stories about Americans traveling to Canada to buy insulin for themselves and their children, thereby skirting around the high cost of this life-giving medicine in the United States. But Canadians, too, are struggling with the rising cost of insulin therapy, as James Elliott has noted. This situation has come about in part because governments have given 100% control of the supply of insulin to global manufacturers, three of which now control 95% of the world market.
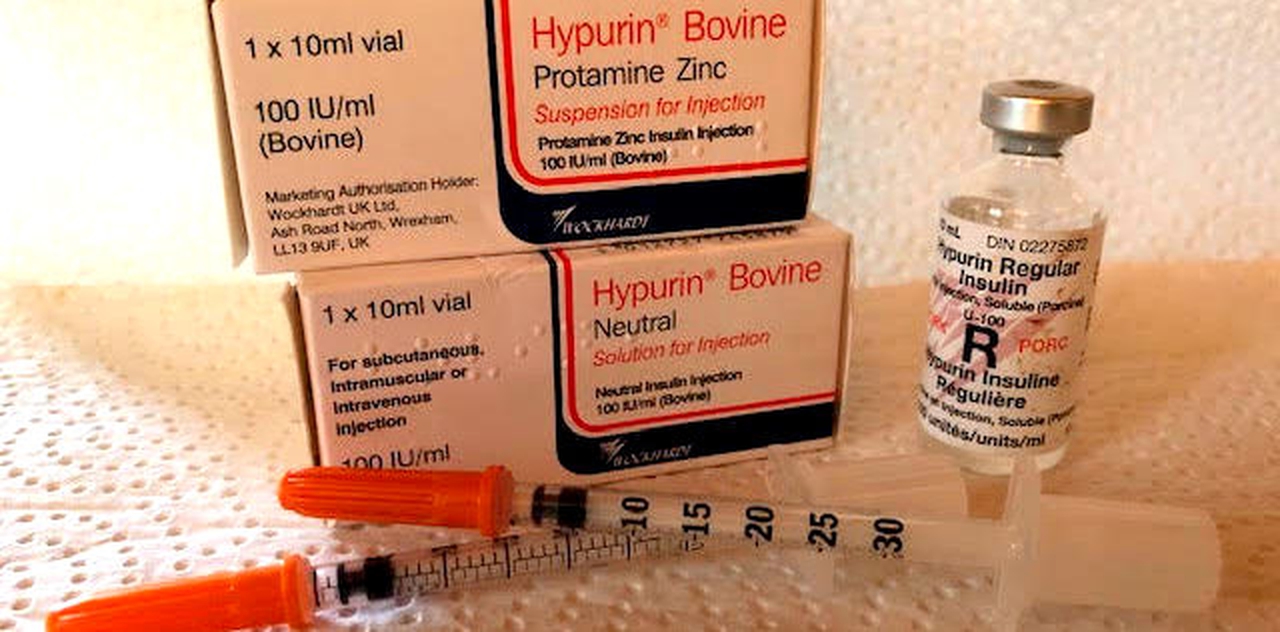
While things have never been perfect, they certainly have been better. Until the mid-1990s, the range of available insulins was much more diverse, not to mention much more affordable. There were about 30 distinct types of insulin including 6-9 different options in the Lente family (Lente, Semilente and the 36-hour, no peak Ultralente), NPH and Regular. Most of these came in beef, pork or a mixed beef/pork formulation. In 1983, the first recombinant (rDNA) human insulin was introduced in North America by Eli Lilly at a price of $18 per 10ml vial, compared to $6 for beef insulin, $6.31 for beef/pork and $11 for pork.
That same year, Novo Nordisk entered the insulin market in Canada through a joint venture with the iconic publicly-owned Connaught Laboratories, where insulin was discovered. Under the agreement, animal insulin would be manufactured in Canada by Novo Nordisk and distributed by Connaught. In 1985 Connaught was privatized and at the end of the decade was sold to what is now Sanofi, ending over sixty years of insulin manufacturing in Canada and turning the country into a net importer of this essential medicine.
There was great reluctance among many people with type 1 diabetes to switch from animal insulin to rDNA human insulin. In part, this was because the best that could be said of rDNA insulin was that it was no better than its animal-sourced predecessor, but it cost between 70% and 300% more. There also were troubling reports of serious adverse reactions to recombinant human insulins. Nonetheless by 2001, 25 types of animal-sourced insulin had been pulled from Canada by manufacturers, leaving more than 40,000 people scrambling to find safe and affordable alternatives. In 1997, the Canadian Diabetes Association (now Diabetes Canada) reported [1] that a survey of members found that “a significant number reported they had problems” when they switched to rDNA human insulin: 43% said the move to rDNA insulin was “not easy,” while 37% said they would change insulins “given the chance.”
Many people reported that the new insulins were less stable, with sharper and more unpredictable peaks. Internationally, there were reports of unexpectedly severe hypoglycaemia linked to the disappearance of early warning signals, and of immunological side effects, including what Eli Lilly referred to as “a syndrome consisting of arthralgia, arthritis, myalgia” [2]. These adverse reactions led to attempted class action lawsuits against manufacturers in Canada, the U.S. and Britain during the 1990s. By 1995, Humulin insulin was among the top 10 drugs in the U.S. linked to serious adverse effects, including death [3]. By 2011, insulin was among the top five drug classes most likely to cause adverse events, reflecting a seismic shift in insulin’s historic track record of safety and effectiveness.
In the mid-1990s, the two companies began to withdraw animal insulin from the worldwide market, thereby removing an important gold standard comparator and drastically (and dangerously) narrowing the range of options available to people who needed insulin. This wasn’t the first time a manufacturer had pulled insulin from the market. In 1985, Eli Lilly was the sole producer of insulin in Argentina when the country was in the grips of an economic crisis. In response to inflation rates above 800%, the government implemented price controls on all products, including medicines. Eli Lilly, in response to “government-controlled pharmaceutical prices” closed its plant, leaving 73,500 people stranded without access to insulin, until the government established a domestic producer to meet their needs.
This is history we should pay attention to, especially now that Canada has moved to strengthen its control over drug prices, a move that is aggressively opposed by the industry. People who need insulin to live will have to push back to protect access, but we also may want to consider “re-domesticating” and diversifying insulin production to protect ourselves from global producers.